Brain Cell Damage From Diet Soda
The artificial dipeptide sweetener, aspartame (APM; L-aspartyl-L-phenylalanine methyl ester), is present in many products in the market, especially in unsweetened or sugar-free products. People trying to lose weight or patients with diabetes, including children, frequently use these products. A recent observation indicated that aspartame is slowly making its way into ordinary products used every day, which do not carry any indication of being for people on diets or diabetics. Thus, aspartame is used not only by the above-mentioned group of people, but also by unsuspecting individuals. Although there is concern and research evidence suggesting possible adverse neurological and behavioural effects due to aspartame's metabolic components (phenylalanine, aspartic acid (aspartate), diketopiperazine and methanol), which are produced during its breakdown, research suggests that aspartame is not cytotoxic. This debate still continues 20 years after the FDA had approved the use of aspartame. As seen later in the literature study, phenylalanine may cross the blood–brain barrier and cause severe changes in the production of very important neurotransmitters. Methanol breaks down into formate, which in turn is very cytotoxic and can even cause blindness.
The effects of aspartame have been studied on various species, including humans, rats, mice and rabbits. Most studies described in the literature have a macroscopic approach. If no adverse effects are visible after a single large administered dose of aspartame, it is believed that aspartame has no effect. Further studies are not carried out microscopically to demonstrate possible adverse effects on the cellular basis. Thus, results obtained from different studies vary from severe adverse effects to none observed.
The aim of this study was to investigate the direct and indirect cellular effects of aspartame on the brain, and we propose that excessive aspartame ingestion might be involved in the pathogenesis of certain mental disorders (DSM-IV-TR 2000) and also in compromised learning and emotional functioning. Most diet beverages and food products currently in the market contain aspartame as an artificial sweetener. However, controversy surrounds the effects of this non-nutritive artificial sweetener, as it is made up of three components that may have adverse effects on neural functioning, particularly on neurotransmitters (Figure 1), neurons and astrocytes.
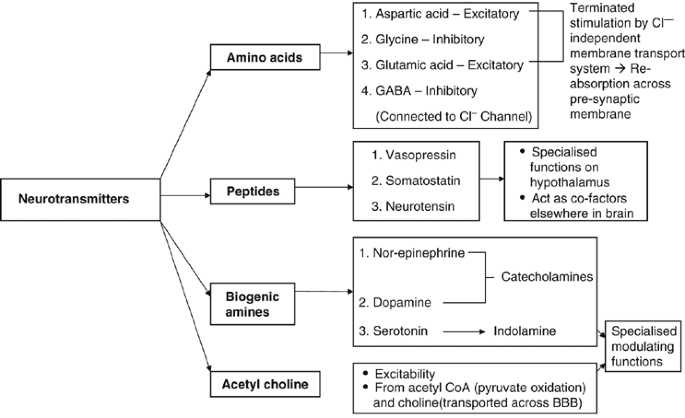
Classification of neurotransmitters.
Full size image
In light of the possible adverse effects of aspartame, the research questions directing this study are formulated as follows: What are the direct and indirect cellular effects of aspartame on the brain? How might excessive aspartame ingestion contribute to the pathogenesis of certain mental disorders? What are the implications for early brain development, emotional status and learning following high ingestion of aspartame?
Aspartame is composed of phenylalanine (50%), aspartic acid (40%) and methanol (10%). The first two are known as amino acid isolates. It has been reported that consumption of aspartame could cause neurological and behavioural disturbances in sensitive individuals (Anonymous, 1984; Johns, 1986). Headaches, insomnia and seizures are some of the neurological disturbances that have been encountered, and this may be accredited to changes in regional brain concentrations of catecholamines, which include norepinephrine, epinephrine and dopamine (Coulombe and Sharma, 1986), all important neurotransmitters regulating life-sustaining functions. The effects of phenylalanine, aspartic acid and methanol are first reviewed, followed by a discussion of altered neurotransmitter functioning, that is dopamine, serotonin, glutamate, γ-aminobutyric acid (GABA), and acetylcholine. The discussion is concluded with implications for early brain development, emotional status and learning following high ingestion of aspartame.
Effects of phenylalanine
Phenylalanine not only plays a role in amino acid metabolism and protein structuring in all tissues, but is also a precursor for tyrosine (Hawkins et al., 1988), DOPA, dopamine, norepinephrine, epinephrine (Ganong, 1997), phenylethylamine (Young, 1988) and phenylacetate (as phenylacetate interferes with brain development and fatty acid metabolism). Phenylalanine also plays an important role in neurotransmitter regulation (Caballero and Wurtman, 1988).
Phenylalanine can follow one of the two pathways of uptake in the body. A part is converted into tyrosine (a non-essential amino acid) in the liver (Caballero and Wurtman, 1988) by the enzyme phenylalanine hydroxylase (Figure 2a) The remaining portion of phenylalanine (not converted in the liver) will bind to a large neutral amino acid transporter (NAAT) to be carried over the blood–brain barrier (BBB) (Figure 2b). A large number of compounds, including phenylalanine and tyrosine, compete with each other for a binding site on the NAAT, because it is the only manner in which they can cross the BBB. Importantly, tyrosine cannot be synthesized in the brain and has have to enter the BBB via NAAT (Figure 2c) for production. Memory loss is thought to be due to aspartic acid and phenylalanine being neurotoxic without the other amino acids found in protein. These neurotoxic agents might cross the BBB and deteriorate the neurons of the brain (Mehl-Madrona, 2005).
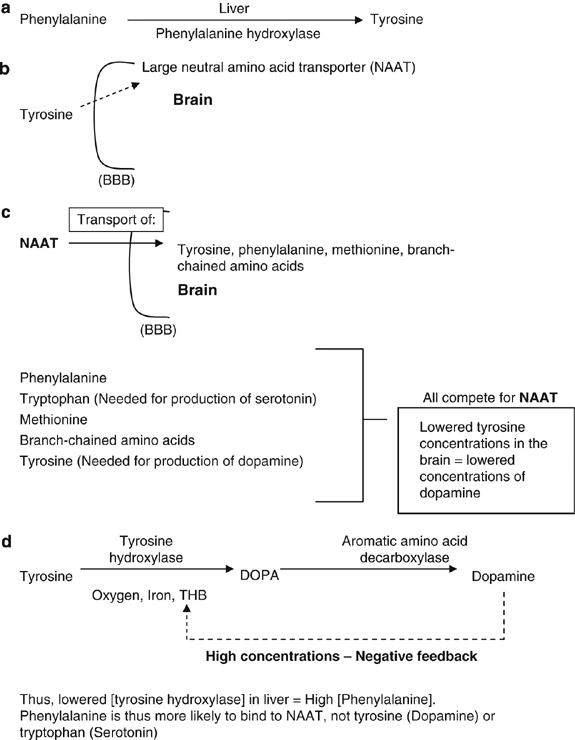
(a) Production of tyrosine from phenylalanine. (b) Transport of tyrosine across the blood–brain barrier (BBB). (c) Co-transporter (NAAT) for transport of tyrosine and so on across the BBB. (d) Conversion of tyrosine into dopamine.
Full size image
NAAT is also a co-transporter for phenylalanine, tryptophan (an important precursor for synthesis of serotonin), methionine and the branch-chained amino acids. All the above-mentioned amino acids (tyrosine, phenylalanine, tryptophan and methionine) compete for the NAAT transporter, so a large quantity of one amino acid in the blood stream will occupy most of this transporter. This results in a phenylalanine overload in the surrounding areas, greatly limiting the amount of important amino acids (for example, tyrosine, tryptophan and methionine) entering the brain (Figure 2c). If high concentration of aspartame is taken through the daily diet, 50% of it is broken down to phenylalanine. Phenylalanine will then be either converted into tyrosine or cross the BBB as it is. Tyrosine is converted into dihydroxyphenylalanine (DOPA) once it is in the brain, by the enzyme tyrosine hydroxylase, with the help of the co-factors oxygen, iron and tetrahydrobiopterin (THB) (Figure 2d).
Dopamine, a catecholamine, is formed from DOPA by an aromatic amino acid decarboxylase. Tyrosine hydroxylase activity is inhibited by high concentrations of dopamine through its influence on the THB co-factor (negative feedback, Figure 2d). This system is very necessary to prevent large amount of dopamine being produced, as dopamine is an inhibitory neurotransmitter. However, if phenylalanine, as the main part of aspartame, competes with tyrosine for NAAT, a compromised dopamine production will result because phenylalanine will bind more frequently and freely than tyrosine owing to its higher concentration, and thus lead to lower concentrations of dopamine in the brain. After administration of aspartame to humans, the increases in blood levels of both phenylalanine and tyrosine have been well documented (Fernstorm, 1988; Filer and Stegink, 1988). Therefore, phenylalanine (formed by breakdown of aspartame) will increase in the brain owing to the ingestion of aspartame, and tyrosine will increase as a breakdown by-product of phenylalanine in the liver (Fernstorm, 1988; Filer and Stegink, 1988). Thus, aspartame and its components could potentially disrupt a wide range of processes in the body, including amino acid metabolism, protein structure and metabolism, nucleic acid integrity, neuronal function and endocrine balances.
Aspartame ingestion directly results in an increase inphenylalanine and tyrosine levels in the brain, which in turn leads to changes in the regional brain concentrations of catecholamines (for example, dopamine) (Fernstorm et al., 1983). According to Mehl-Madrona (2005) aspartame changes the dopamine level in the brain, affecting people suffering from Parkinson's disease. Bowen and Evangelista (2002) noted a substantial increase in the levels of plasma phenylalanine and aspartic acid after ingestion of aspartame. This increased phenylalanine, thereby causing a PKU (phenylketonuria) effect. PKU, also known as phenylpyruvic oligophrenia, is a disorder characterized by accumulation of phenylalanine and its keto derivatives in the blood, tissues and urine. This disorder is a direct result of a hereditary deficiency or absence of phenylalanine hydroxylase. As described previously, this enzyme is necessary for conversion of phenylalanine into tyrosine. The enzymes required for the reduction of circulating phenylalanine are overwhelmed, thus also interfering with other metabolic reactions that utilize these enzymes, resulting in the PKU effect. This causes reduced dopamine and serotonin production as the enzyme actions controlling numerous types of neurotransmitters (and their precursor amino acids) are debilitated by overdoses of the competitive circulating phenylalanine isolates (and aspartic acid isolates; Bowen and Evangelista, 2002).
Serotonin, an indolamine, causes powerful smooth muscle contraction (Ganong, 1997). Physiologically, it is also important for behaviour and control of sleep, temperature, appetite and neuroendocrine functions. Tryptophan, independently utilized for synthesis of serotonin in the brain, is transported across the BBB via NAAT. Therefore, if NAAT is occupied with phenylalanine, tryptophan will not be adequately carried across the BBB and serotonin production can ultimately be compromised (Figure 3).
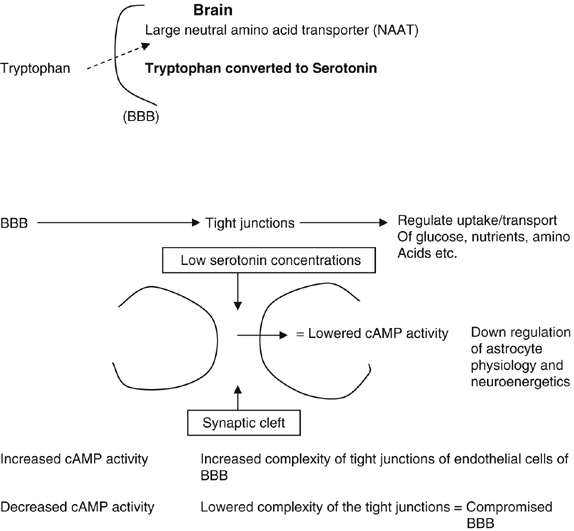
Production of serotonin from tryptophan.
Full size image
Aspartame administered orally in mice as single doses gave contradictory results; norepinephrine and dopamine (precursor of norepinephrine) concentrations in various brain regions increased significantly, and not as observed above. However, mice have a different metabolism for aspartame and its breakdown products are different from those of human beings; this could be the reason for these contradictory results. Sharma and Coulombe (1987) also analysed different regions for catecholamine (for example, dopamine) and indoleamine (for example, serotonin) neurotransmitters and their major metabolites. Results from this study indicated that single dose exposure increased adrenergic chemicals, which were not apparent after repeated dosing with aspartame. In contrast to the above observation, decreased serotonin and its metabolite, 5-hydroxyindoleacetate, was found in several regions (Sharma and Coulombe, 1987). The lowered levels of serotonin might cause the following:
-
A compromised BBB—due to lower levels of activity of cAMP, which plays an important role in the complexity of the tight junctions in the epithelial cells of the capillaries (Figure 3).
-
Lowered activity of the GABA transporters—thus GABA is absorbed at a lower rate into the astrocytes, which results in the continuous inhibition of depolarization of the postsynaptic membrane (Figure 4).
Figure 4 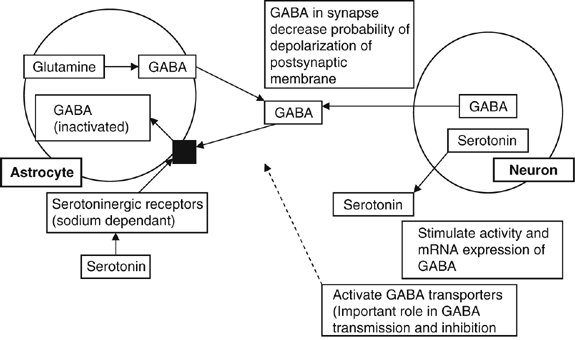
Effects of lowered levels of serotonin on γ-aminobutyric acid (GABA) in the synaptic cleft.
Full size image
Maher and Wurtman (1987) suggested that aspartame consumption could cause neurological or behavioural reactions in some people. When mice were given aspartame in doses that raise plasma phenylalanine levels more than those of tyrosine (which probably occurs after any aspartame dose in humans), the frequency of seizures increased, especially following the administration of the epileptogenic drug, pentylenetetrazole. Equimolar concentrations of phenylalanine stimulate this effect and are blocked by synchronized administration of valine, which blocks phenylalanine's entry into the brain (Maher and Wurtman, 1987).
Glutamate, the most common neurotransmitter in the brain, is formed from its precursor α-ketoglutarate from the Kreb's cycle (Figure 5). Glutamate is primarily produced in neurons as excitatory neurotransmitters owing to an increased flow of positive ions (sodium and calcium) by opening the ion-channel after binding to appropriate receptors. Stimulation of these receptors is terminated by a chloride-independent membrane transport system, which is used only for reabsorbing glutamate and aspartate across the presynaptic membrane. Glutamate can also be reabsorbed into the neurons for later use. Excess glutamate released into the synapses is converted into glutamine (non-excitotoxic molecule) by nearby astrocytes (glial cells). Glutamine is safely transported back to neurons, for reconversion into glutamate. Swollen astrocytes contribute to the excitotoxicity of glutamate owing to their inability to absorb excess glutamate. Glutamate acts on its postsynaptic N-methyl-D-aspartate (NMDA) and non-NMDA receptors. The NMDA receptor is an ion channel for calcium, sodium and potassium ions. Glutamate and aspartate exert their action through three separate receptors characterized by selective interaction with NMDA, quisqualate and kainate (Hidemitsu et al., 1990). The glutamate recognition sites might directly be acted upon by aspartame in the brain synaptic membranes. This interaction might play a vital role in mediating the potentiation of hippocampal excitability as reported by Fountain et al. (1988).
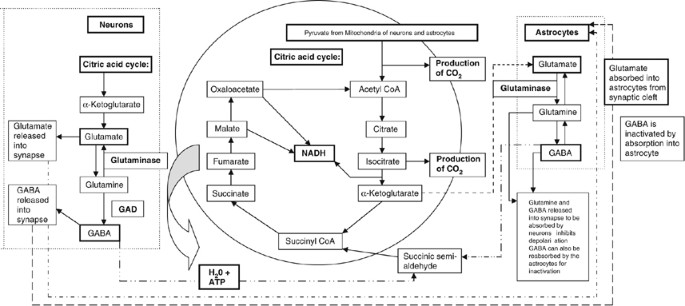
Production pathway of glutamate and γ-aminobutyric acid (GABA) in neuronal and glial cells.
Full size image
As discussed above, aspartame may act on the NMDA receptors, leading to continuous activation of these receptor sites resulting in no binding space for glutamate. Continuous activation might cause damage to brain neurons, as suggested by Choi and Rothman (1990). Thus, aspartame acts as an agonist of glutamate on the NMDA receptor (Fountain et al., 1988).
GABA is also primarily produced by neurons in the citric acid cycle from succinate and is inactivated by absorption into astrocytes (Figure 5). GABA is secondarily produced in astrocytes from glutamine. It can be released from the astrocytes as GABA or it can be reabsorbed into the neuron as glutamine (for conversion into either glutamate or GABA). If the neuroenergetics of the cells were compromised by the presence of aspartame, thus lowering glucose and oxidative metabolism, this important feedback system of tryptophan and tyrosine will be inhibited (Ganong, 1997).
Owing to a lowered level of oxidative metabolism and low glucose levels in the cells, pyruvate would not be converted into acetyl CoA necessary for production of acetylcholine in synapses (Figure 6). Thus, it could lead to a decreased stimulation of second messengers (often cyclic AMP) to indirectly open the ion channels. Since aspartame causes neurodegeneration (destructions of neurons), the neurons in the Meynert nucleus will also be decreased. The Meynert nucleus is the primary cholinergic input for the cerebral cortex, and loss of neurons in this nucleus has been shown in Alzheimer's patients. Thus, aspartame might be involved in the cause/mimic of Alzheimer's disease (Ganong, 1997; Bowen and Evangelista, 2002).
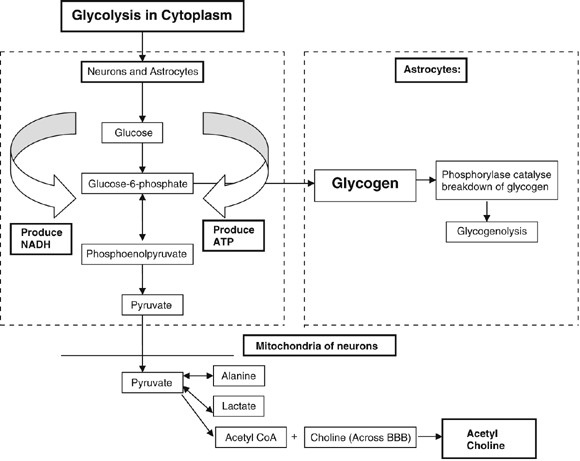
Production pathway of acetylcholine in neuronal cells.
Full size image
Effects of aspartic acid
One of the largest studies commissioned by the aspartame manufactures are of the opinion that: 'in most cases aspartate concentrations were not significantly affected by aspartame ingestion' (Stegink et al., 1988; Stegink et al., 1989). If read in another way, it suggests that in some cases aspartic acid was, indeed, increased. Aspartic acid is thought to play a role as an excitatory neurotransmitter in the central nervous system (Watkins, 1984; Stone and Burton, 1988). Glutamate, asparagines and glutamine are formed from their precursor, aspartic acid (Stegink et al., 1989). Aspartate is inactivated by reabsorption into the presynaptic membrane and it opens an ion channel (Olney, 1975). Aspartate is an excitatory neurotransmitter and has an increased likelihood for depolarization of the postsynaptic membrane. Even short-lived increases of a powerful neural stimulator are enough to induce neuroendocrine disturbances (Olney, 1975). In addition, Mehl-Madrona (2005) observed that when the temperature of aspartame exceeds 86°F, the wood alcohol in aspartame is converted into formaldehyde and then to formic acid, which in turn causes metabolic acidosis. The methanol toxicity is thought to mimic the symptoms of multiple sclerosis. According to them, symptoms of fibromyalgia, spasms, shooting pains, numbness in the legs, cramps, vertigo, dizziness, headaches, tinnitus, joint pain, depression, anxiety, slurred speech, blurred vision or memory loss have been attributed to aspartame.
Effects of methanol
As mentioned previously, aspartame breaks down to form phenylalanine, aspartic acid and methanol, which form 10% of the break down product. The methanol in the body is converted to formate, which is then excreted. It can also give rise to formaldehyde, diketopiperazine (a carcinogen) and a number of other highly toxic derivatives (Clarke, 2000). The absorption-metabolism sequence of methanol → formaldehyde → formic acid also results in synergistic damage (Bowen and Evangelista, 2002). The accumulation of formate rather than methanol is itself considered to cause methanol toxicity (Stegink et al., 1989), but research has shown that formaldehyde adducts accumulate in the tissues, in both proteins and nucleic acids, after aspartame ingestion (Trocho et al., 1998). The formed adducts of the metabolic poisons alter both mitochondrial DNA and nucleic DNA. Methanol and formaldehyde are also known to be carcinogenic and mutagenic. The damaged DNA could cause the cell to function inadequately or have an unbalanced homoeostasis, thus initiating disease states (Bowen and Evangelista, 2002). In addition, it is thought that the methanol is the aspartame is converted to formaldehyde in the retina of the eye, causing blindness (Mehl-Madrona, 2005).
As seen from the above discussion, tryptophan, tyrosine and phenylalanine are precursors for the neurotransmitters serotonin, dopamine and norepinephrine. Glutamate (glutamic acid) and aspartate (aspartic acid), as neurotransmitters, have no direct access to the brain and have to be synthesized in the neuronal cells of the brain. Proteins rich in aspartate and glutamate have no effect on the levels of acidic amino acids in the brain. If aspartame is ingested in large amounts, it will increase the levels of acidic amino acids in the brain (Fernstrom, 1994).
Effects of aspartame on the blood brain barrier
A compromised BBB (altered lipid-mediated transport or active carrier transport) will result in the transport of excitotoxins (aspartame) across BBB and within the cerebrospinal fluid causing several adverse reactions to occur:
-
The nerves will be stimulated to fire excessively by the excitotoxins.
-
The offset of induced, repeated firing of the neurons mentioned above will require normal enzymes, which are negated by the phenylalanine and aspartic acid present in aspartame.
These compulsory enzyme reactions mentioned above require a normal functioning energy system. Thus, it could be stated that the neurons become compromised from (Bowen and Evangelista, 2002):
-
diminishing intracellular ATP stores;
-
the presence of formaldehyde;
-
intracellular calcium uptake been changed (e.g. phenylalanine binds to NMDA receptor, not glutamate, thus altering calcium channels);
-
cellular mitochondrial damage;
-
destruction of the cellular wall; and
-
subsequent release of free radicals.
These preceding reactions potentiate oxidative stress and neurodegeneration. Secondary damage is caused by the toxic by-products, which in turn will increase capillary permeability, continuing to destroy the surrounding nerve and glial cells, thus further obstructing enzyme reactions and promoting DNA structural defects. Cellular death occurs over the next 1–12 h (Bowen and Evangelista, 2002).
Excitotoxic-saturated placental blood flow, caused by maternal aspartame consumption, could lead to the damage or impairment of the development of the foetal nervous system, contributing to cerebral palsy and all-encompassing developmental disorders (Bowen and Evangelista, 2002). Mehl-Madrona (2005) also cited findings implicating aspartame consumption at the time of conception to consequent birth defects, because the phenylalanine concentrates in the placenta, causing mental retardation. Laboratory tests showed that animals developed brain tumours as a result of aspartame administration. It was also pointed out that phenylalanine breaks down into 1-deoxy-D-xylulose-5-phosphate (DXP), a brain tumour agent. In keeping with these findings, neuronal (brain) damage is also produced by excitotoxins circulating in the fetal brain areas, as a result of an incompetent BBB. This is especially true for those areas adjacent to the brain's ventricular system. The methanol components of aspartame are thought to mimic fetal alcohol syndrome, which is a direct result of the maternal ingestion of aspartame (Bowen and Evangelista, 2002).
The amino acids that constitute meat contain a chain of 80–300 amino acids, of which 4% are phenylalanine. This chain also includes the amino acid valine. Valine inhibits the transport of phenylalanine into the brain across the BBB. In aspartame, phenylalanine makes up 50% of the molecule; thus, in a can of diet soda, which contains 200 mg aspartame, 100 mg is phenylalanine. No valine is present in aspartame to block the entry of toxic levels of phenylalanine into the brain, thus resulting in lowered concentrations of dopamine and serotonin owing to NAAT occupation by phenylalanine.
Thus, it can be concluded that the usage of aspartame should be carefully considered as it (and its metabolites) causes detrimental effects, ranging from alterations in concentrations of neurotransmitters to causing infertility. Thus, human health at the macroscopic, microscopic and cellular level is at risk of being destroyed.
Comparison between human and animal reaction to aspartame
Physiologically, the animals tested for phenylalanine toxicity are approximately 60 times less sensitive than human beings. Humans are 10–20 times more sensitive to methanol poisoning both as a subchronic and chronic toxin/carcinogen. The differences in enzyme concentrations of the species suggest that animals studied are more sensitive to the more common ethanol found in alcoholic beverages. Test animals being used are 8–10 times less sensitive than humans to the effects of aspartic acid and glutamates (Bowen and Evangelista, 2002).
Implications of aspartame consumption for early brain development and everyday living
Ingestion of aspartame results in a craving for carbohydrates, which will eventually result in weight gain, especially because the formaldehyde stores in the fat cells, particularly in the hips and thighs; therefore, aspartame is believed to cause problem in diabetic control. (Mehl-Madrona, 2005). In addition, prenatal consumption of aspartame might result in mental retardation, impaired vision, birth defects and is thought to play a role in the pathogenesis of Alzheimer's disease; furthermore, it is implicated in disruption of learning and emotional functioning due to its involvement in alteration of certain neurotransmitters. The earlier research findings show that aspartame consumption might affect early brain development and neurotransmitter systems, which might result in specific emotional, behavioural and learning difficulties as discussed below.
Dopamine involvement in emotional status and learning
In the preceding sections it was noted that when phenylalanine, one of the main component of aspartame, competes with tyrosine for NAAT, a compromised dopamine production will result, because phenylalanine will bind more frequently and freely than tyrosine owing to its higher concentration. This will thus lead to lower concentrations of dopamine in the brain. Dopamine receptors are numbered D1, D2, D3, D4 and D5 receptors, all playing an important role in the dopaminergic system. The dopaminergic system is active in maintaining normal motor behaviour, and loss of dopamine is related to Parkinson's disease, in which the muscles are rigid and movement is difficult (Kolb and Whishaw, 2003). Disturbances of the development of the dopaminergic system may lead to dyskinesia, dystonia, tics, obsessive–compulsive disorders and abnormal eye movements (Herlenius and Langercrantz, 2004). This has been observed in DA-depleted rats after 6-hydroxyl dopamine treatment but with preserved noradrenaline effect (Zhou and Palmiter, 1995). D1-receptors are involved in working memory performance (Williams and Goldman-Rakic (1995)). A disturbance of the development of the dopaminergic system has been postulated to contribute to the cause of attention deficit hyperactivity disorder (ADHD) in which a deficient working memory is an important component (Dare et al., 2003). In 2002, Bowen and Evangelista noted a substantial increase in levels of plasma phenylalanine and aspartic acid after ingestion of aspartame. This increased phenylalanine causes PKU effect as noted earlier in this study. Infants with phenylketonuria and probably deficient dopaminergic innervation of the prefrontal cortex have been found to have (among other symptoms) an impaired working memory (Diamond et al., 2004).
Serotonin involvement in early brain development, emotional status and learning
Tryptophan, independently utilized for synthesis of serotonin in the brain, is transported across the BBB via NAAT. Therefore, if NAAT is saturated with phenylalanine, tryptophan will not be adequately carried over the BBB and serotonin production can ultimately be compromised. In addition to its role in regulating maturation of terminal areas, serotonin can set its own terminal density—a phenomenon Whitaker-Azmitia (2001) termed autoregulation of development.
Serotonin (5-HT), like other monoamine neurotransmitters, has been shown to play a role in regulating brain development before the time it assumes its role as a neurotransmitter in the mature brain (Chubakov et al., 1986, 1993; Lauder, 1990; Whitaker-Azmitia, 1991; Turlejski, 1996; Whitaker-Azmitia et al., 1996). This neurotransmitter is concentrated in the raphe nucleus of the brain, and is also present in platelets. Serotonin and serotonergic neurons are localized in the midbrain, the pineal gland, the substantia nigra, the hypothalamus and the raphe nuclei of the brain stem (Herlenius and Lagercrantz, 2004). The 5-HT neurons have widespread projections, making it possible to coordinate complex sensory and motor behavioural conditions. Serotonin is also involved in inducing sleep, sensory perception, temperature regulation and control of mood; therefore, serotoninergic activity was found to be highest during waking and arousal and absent during active or rapid eye-movement sleep (Boutrel et al, 1999).
In addition, serotonin has been reported to affect neuronal proliferation, differentiation, migration and synaptogenesis (Gaspar et al., 2003). In the mammalian brain, all the monoamine neurotransmitter systems are present relatively early but, in particular, serotonin is likely to present the earliest in the most terminal regions (Whitaker-Azmitia, 2001). These early appearances of serotonergic neurons with their wide distribution of terminals play a crucial role in programmed neurogenesis, synaptogenesis and apoptosis. Serotonergic cells in the raphne are among the earliest to be generated in the brain (Gaspar et al., 2003). Therefore, serotonin concentration must be neither too high nor too low during the critical period of synaptogenesis and formation of cortical connections. Serotonergic abnormalities are also associated with abnormalities of cortical development and thalamocortical connectivity, as abnormal serotonin transport or synthesis during brain development may directly affect formation of intracortical and thalamocortical circuitry (Chugani, 2004). Furthermore, disruptions of the serotonergic pathways due to excess or inadequate activation of specific 5-HT receptors during development are implicated in the pathogenesis of developmental disorders such as autism (Gaspar et al., 2003). The relative balance of tryptophan metabolism, regulated by the serotonin and kynurenine pathways, might therefore be important in the pathogenesis of pervasive developmental disorders among children, and aspartame consumption may therefore play a role in the occurrence of developmental disorders.
GABA involvement in early brain development, emotional status and learning
The removal of the carboyxl (COOH) group from glutamate produces GABA, which is the main inhibitory transmitter (Kolb and Whishaw, 2003), and perhaps 25–40% of all nerve terminals contain GABA (Herlenius and Lagercrantz, 2004). In humans, the majority of neocortical GABAergic neurons arise locally in the ventricular and subventricular zone. Proportionally fewer GABAergic neurons originate from the ganglionic eminence of the ventral forebrain (Letinic et al., 2002). The lowered levels of serotonin due to aspartame consumption might cause lowered activity of the GABA transporters, and thus GABA is absorbed at a lower rate into the astrocytes, which will result in the continuous inhibition of depolarization of the postsynaptic membrane.
Although GABA is regarded as the main inhibitory transmitter in the mature animal, it has a different role during early development (Herlenius and Lagercrantz, 2004). During early brain development, it acts as a trophic factor to influence events such as proliferation, migration, differentiation, synapse maturation and cell death (Owens and Kriegstein, 2002). Herlenius and Lagercrantz (2004) report that GABA is a crucial transmitter for the human infant and operates mainly as an excitatory transmitter on immature neurons. As GABA has a trophic role during early brain development, interference with the function of GABAergic transmission during this period may affect the development of neuronal wiring, plasticity of neuronal network and also have a profound influence on neural organization (Herlenius and Lagercrantz, 2004).
Acetylcholine involvement in early brain development, emotional status and learning
Previously, it was mentioned that aspartame could cause changes to acetylcholine production. It is known that at a lowered level of oxidative metabolism and low glucose levels in the cells, pyruvate would not be converted into acetyl CoA necessary for production of acetylcholine in synapses. Acetylcholine is one of the major neurotransmitters of importance in the brain for cortical activation, attention, reward and pain. The cholinergic system is thought to play a role in memory and learning by maintaining neuron excitability. Death of acetylcholine neurons and decrease in acetylcholine in the neocortex are thought to be related to Alzheimer's disease (Kolb and Whishaw, 2003), as it has a major role in the control motor tone and movement and probably counterbalances the effect of dopamine (Johnston and Silverstein, 1998; Cooper et al., 2003). In addition, acetylcholine is of major importance for the development and the control of autonomic functions, and alterations to the cholinergic system might result in major changes in cortical structure. These changes can be correlated to cognitive deficits but do not affect motor behaviour (Herlenius and Lagercrantz, 2004).
Norepinephrine involvement in emotional status and learning
Aspartame may also cause a change in norepinephrine. Compared with dopamine systems, which restrict their outputs to the reptilian brain (that is, the basal ganglia) and frontal cortex, the projections of the caudally situated noradrenaline systems are more widespread. The cell bodies of the noradrenergic neurons are concentrated in the brain stem, particularly in the locus coeruleus within the caudal pons (Kolb and Whishaw, 2003). Five major noradrenergic tracts originate from the locus coeruleus that disperse through the whole brain. There are also clusters of noradrenergic cell bodies in the nucleus tractus solitarius and in the lateral ventral tegmental field (Herlenius and Lagercrantz, 2004). Fibres from these nuclei intermingle with those from the locus coeruleus. The A6 noradrenaline cell group, well known as the locus coeruleus, controls higher brain activity through the dorsal noradrenaline pathway. This group sends inputs to the cortex, hypothalamus, cerebellum, lower brain stem and spinal cord, thereby exerting control over cortical arousal and attention, fear and anxiety, and learning and memory. The ventral noradrenaline pathway infiltrates the hypothalamus and the limbic system (Panksepp, 1998).
Noradrenergic neurons appear at an early stage in the development of the central nervous system. Sundstrom et al (1993) reported noradrenergic neuronal development at the 12th to 14th day of gestation in the rat and within 5-6 weeks in the human, and Sundstrom (1996) later suggested that noradrenaline is essential for normal brain development. In addition, the noradrenergic system regulates the development of the Cajal-Retzius cells that are the first neurons to be formed in the cortex (Herlenius and Lagercrantz, 2004). Wang and Lidow (1997) showed that radial glia participate in key steps of brain development and cortical neurogenesis, whereas two independent studies showed glia participation in migration (Noctor et al., 2001, 2004). Thus, adrenergic transmission may be involved in regulating the generation, migration and maturation of cerebral cortical cells. Herlenius and Lagercrantz (2004) reported that administration of 6-OH-dopamine prevents programmed cell death of these neurons and delays the formation of cortical layers. Lesioning of the noradrenergic projections or blocking of neurotransmission with receptor antagonist prevents astrogliosis and glial cell proliferation.
During postnatal development, noradrenaline plays an important role in regulating attention, as noradrenergic cells are exquisitely sensitive to environmental stimuli, especially powerful emotional events (Panksepp, 1998). With low noradrenaline activity, individuals tend to perseverate on a task despite changes in stimulus contingencies because of attention deficits. Such individuals are prone to act impulsively rather than deliberately. Depletion of noradrenaline during the perinatal period can also result in subtle dendritic changes and possibly also alterations in cortical differentiation that may lead to behavioural changes (Berger-Sweeney and Hohmann, 1997). It is also known that noradrenaline dampens the background 'noise' or cortical neural activity irrelevant to a given task (Panksepp, 1998). This makes the influence of specific incoming signals more prominent in the cortex, namely the ratio of the signal to background noise is increased. Thus, it is suspected that with high noradrenaline activity, individuals can better process information that already has access to the cortex.
Glutamate involvement in emotional status and learning
The glutamate recognition sites might directly be acted upon by aspartame in the brain synaptic membranes, and aspartame may act on the NMDA receptors, leading to continuous activation of these receptor sites and no binding space for glutamate. The excitatory amino acid transmitter glutamate and the inhibitory amino acid transmitter GABA are closely related in the sense that GABA is formed by a simple modification of glutamate (Herlenius and Lagercrantz, 2004). Glutamate is widely distributed in the forebrain and cerebellum and also in neurons, but it becomes a neurotransmitter only if it is appropriately packed in vesicles in the axon terminal (Kolb and Whishaw, 2003). Glutamate acts on at least five types of receptors, and particularly the NMDA receptors dominate in the immature brain when synaptic transmission is weak and extremely plastic, as the NMDA receptors permit entry of Na+ and Ca2+ when opened. NMDA channels seem to be crucially involved in the appearance of long-term potentiation and synaptic plasticity underlying learning and memory storage throughout life (Herlenius and Lagercrantz, 2004). Cell death resulting from glutamate occurs in two ways: first, it causes an increase in intracellular calcium that poisons the cell, and second, the increase in intracellular calcium can activate genes in the cell's DNA to produce proteins that kill the cell, called apoptosis (Kolb and Whishaw, 2003). During critical periods of development and synaptogenesis, NMDA receptors play an essential role in activity-dependent plasticity and synaptic refinement (McDonald and Johnston, 1990; Qu et al., 2003). Thus, either too much or too little NMDA receptor activity can be life-threatening to developing neurons (Lipton and Nakanishi, 1999).
Brain Cell Damage From Diet Soda
Source: https://www.nature.com/articles/1602866
0 Response to "Brain Cell Damage From Diet Soda"
Post a Comment